Chapter 3 – Molecular scale interactions
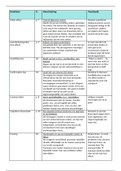
1. Molecular scale:
Attractive forces are a necessity to have condensed phases. You
could never have a solid or liquid phase, when there are no
attractive forces. You could only have gas phases.
2. Macromolecular scale:
Interactions on a macromolecular scale are important in the thickening
behavior of coil shaped polymer chains.
o There can be repulsion between the segment of a thickening agent, and
attraction between the segments & water. There is swelling of the coil, the
volume increases and due to overlapping of the segments, the viscosity
increases: thickening effect.
o Also, the opposite can take place: there is an attraction between the
segments and there is a weak interaction between the segments and
water. The colloidal has a collapsed form and it’ll not enhance the viscosity
of the fluid.
3. Colloidal scale:
Interactions on colloidal scale are important for the stability of emulsions and for
the strength & network formation in products like margarine.
o Emulsion: stability is affected by the balance between attractive and repulsive
forces.
o The interaction between two droplets is strongly repulsive, because
otherwise aggregation would take place.
o Margarine: a product like margarine is solid due to the formation of a network of fat
crystals, driven by attraction forces between the crystals.
All interactions that we know in nature, can be reduced to 4 fundamental forces:
1. Weak interactions in the core of atoms.
2. Strong interactions in the core of atoms.
3. Gravitational forces.
4. Electromagnetic forces. (The interactions we’ll focus on below, are derived from
electromagnetic forces).
- Electromagnetic forces: interactions between charges.
1. Permanent charge (PC):
Ions have a permanent charge; they continuously have either a positive or a
negative charge.
2. Permanent dipole (PD):
Permanent dipoles are molecules that have a net zero charge, but the center of mass of
the positive charges does not go inside with the center of mass of the negative charges.
o 1 side is slightly positively charged, 1 side is slightly negatively charged.
, o The cause for this, is that atoms in the molecule have a different
electronegativity.
3. Induced dipole (ID):
Molecules that, in absence of charge, are completely uncharged. However,
when they are in contact with a permanent charge, 1 side will turn
slightly positively charged, one side will turn slightly negatively
charged.
There are 3 possible dipole-dipole interactions (in orange); together we call this van der
Waals interactions.
The strength of an interaction can be characterized by the
interaction potential V(r) and the interaction force F(r):
- Interaction Potential V(r): the energy required to take 2 particles,
which are at infinite separation, to bring them to a distance r.
- Interaction Force F(r): this force is related to the interaction potential; it is the negative of
the derivative of the potential, with respect to r.
r
−dV (r ) ' '
F ( r )= V ( r )=−∫ F ( r ) d r
dr ∞
o V(r) is thus the integral of F(r).
o Repulsion: F > 0.
o Attraction: F<0.
Another important property of an interaction is
the effective range of an interaction.
- Effective range: the point where the potential
drops below the thermal energy. Below that point,
molecules do no longer sense the interaction energy
and the system is dominated by thermal energy.
In the graph on the right, the potential is plotted
as a function of r. It’s is a decreasing function with
distance. Repulsive.
Apart from the interaction energy, all molecules in a system also experience thermal
energy: V thermal =k B∗T .
T: absolute temperature (K).
1. Molecular scale:
Attractive forces are a necessity to have condensed phases. You
could never have a solid or liquid phase, when there are no
attractive forces. You could only have gas phases.
2. Macromolecular scale:
Interactions on a macromolecular scale are important in the thickening
behavior of coil shaped polymer chains.
o There can be repulsion between the segment of a thickening agent, and
attraction between the segments & water. There is swelling of the coil, the
volume increases and due to overlapping of the segments, the viscosity
increases: thickening effect.
o Also, the opposite can take place: there is an attraction between the
segments and there is a weak interaction between the segments and
water. The colloidal has a collapsed form and it’ll not enhance the viscosity
of the fluid.
3. Colloidal scale:
Interactions on colloidal scale are important for the stability of emulsions and for
the strength & network formation in products like margarine.
o Emulsion: stability is affected by the balance between attractive and repulsive
forces.
o The interaction between two droplets is strongly repulsive, because
otherwise aggregation would take place.
o Margarine: a product like margarine is solid due to the formation of a network of fat
crystals, driven by attraction forces between the crystals.
All interactions that we know in nature, can be reduced to 4 fundamental forces:
1. Weak interactions in the core of atoms.
2. Strong interactions in the core of atoms.
3. Gravitational forces.
4. Electromagnetic forces. (The interactions we’ll focus on below, are derived from
electromagnetic forces).
- Electromagnetic forces: interactions between charges.
1. Permanent charge (PC):
Ions have a permanent charge; they continuously have either a positive or a
negative charge.
2. Permanent dipole (PD):
Permanent dipoles are molecules that have a net zero charge, but the center of mass of
the positive charges does not go inside with the center of mass of the negative charges.
o 1 side is slightly positively charged, 1 side is slightly negatively charged.
, o The cause for this, is that atoms in the molecule have a different
electronegativity.
3. Induced dipole (ID):
Molecules that, in absence of charge, are completely uncharged. However,
when they are in contact with a permanent charge, 1 side will turn
slightly positively charged, one side will turn slightly negatively
charged.
There are 3 possible dipole-dipole interactions (in orange); together we call this van der
Waals interactions.
The strength of an interaction can be characterized by the
interaction potential V(r) and the interaction force F(r):
- Interaction Potential V(r): the energy required to take 2 particles,
which are at infinite separation, to bring them to a distance r.
- Interaction Force F(r): this force is related to the interaction potential; it is the negative of
the derivative of the potential, with respect to r.
r
−dV (r ) ' '
F ( r )= V ( r )=−∫ F ( r ) d r
dr ∞
o V(r) is thus the integral of F(r).
o Repulsion: F > 0.
o Attraction: F<0.
Another important property of an interaction is
the effective range of an interaction.
- Effective range: the point where the potential
drops below the thermal energy. Below that point,
molecules do no longer sense the interaction energy
and the system is dominated by thermal energy.
In the graph on the right, the potential is plotted
as a function of r. It’s is a decreasing function with
distance. Repulsive.
Apart from the interaction energy, all molecules in a system also experience thermal
energy: V thermal =k B∗T .
T: absolute temperature (K).