What is the lar - Study guides, Class notes & Summaries
Looking for the best study guides, study notes and summaries about What is the lar? On this page you'll find 478 study documents about What is the lar.
Page 4 out of 478 results
Sort by

-
Texas Property & Casualty License questions with correct answers
- Exam (elaborations) • 15 pages • 2023
- Available in package deal
-
- $15.49
- + learn more
Property vs Casualty: What is considered property? CORRECT ANSWER Structure & Personal Property Property vs Casualty: What is considered casualty? CORRECT ANSWER Non-property losses. Legal liability to others. 3rd Party Risk CORRECT ANSWER uncertainty of financial loss Pure Risk CORRECT ANSWER Chance of Loss Speculative Risk CORRECT ANSWER Chance of Loss or Gain (Gamble) Insurance (covers which type of risk?) CORRECT ANSWER Pure Risk Only How does the law of large numbers apply...

-
SOCRA Test practice Questions and Answers 2024
- Exam (elaborations) • 22 pages • 2024
-
- $13.49
- + learn more
SOCRA Test practice Questions and Answers 2024 The Purpose of the IRB is to: -Answer-Protect the rights and welfare of human subjects in research What is the minimum number of members required by an IRB -Answer-5 Which of the following are necessary to waive consent? A.Subject is unable to give consent B.No time or unable to contact next of kin C.Life-Threatening Condition D.No other treatment available E.All of the above -Answer-all of the above This form is used for the mandatory rep...

-
CCRP Exam Verified Questions And Answers Graded A 2024
- Exam (elaborations) • 30 pages • 2024
-
Available in package deal
-
- $14.49
- + learn more
How many days does a sponsor have to report an emergency use of an IP to the FDA? - 5 working days How many members must sit on an IRB? - 5 How long must an IRB retain records per 21 CFR 56? - 3 years after completion of research What are the criteria for IRB approval of research? (7) - 1. Risks to subjects are minimized 2. Risks are reasonable in relation to anticipated benefits 3. Selection of subjects is equitable 4. Informed consent will be sought from subjects or LARs 5. Infor...
-
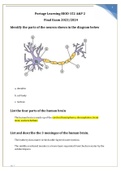
Portage Learning BIOD 152 A&P 2 Final Exam
- Exam (elaborations) • 31 pages • 2023
-
- $10.99
- 2x sold
- + learn more
Portage Learning BIOD 152 A&P 2 Final Exam 2023/2024 Identify the parts of the neuron shown in the diagram below a. dendrite b. cell body c. nucleus List the four parts of the human brain The human brain is made up of the cerebral hemispheres, diencephalon, brain stem, andcerebellum. List and describe the 3 meninges of the human brain. The leathery dura mater is the double-layered outer meninx. The middle arachnoid meninx is a loose layer separated from the dura...

-
FTCE- Elementary Education (Florida Teacher Certification Examination) Comprehensive 100% Answered
- Exam (elaborations) • 104 pages • 2023
- Available in package deal
-
- $16.49
- + learn more
The reading process is made up of these five components: - ANSWER-Phonemic Awareness, Fluency, Phonics, Comprehension, and Vocabulary. LAR Skill 1.1 Components of Emergent Literacy are: - ANSWER-Print Awareness, Print Motivation, Oral Language, Letter Knowledge, Phonological awareness, Narrative skills. LAR Skill 1.1 Emergent Literacy - ANSWER-Consists of reading-related knowldge and skills that children develop prior to formal instruction in reading. LAR Skill 1.1 Print Motiva...

-
CCRP Definitions (21 CFR 50) Human subject Protection requirements Questions and Answers 2023/2024
- Exam (elaborations) • 31 pages • 2024
-
Available in package deal
-
- $14.49
- + learn more
CCRP Definitions (21 CFR 50) Human subject Protection requirements Questions and Answers 2023/2024 21 CFR 50 - correct answer Protection of Human Subjects Sponsor - correct answer a person who initiates a clinical investigation, but who does not actually conduct the investigation Sponsor-investigator - correct answer an individual who both initiates and actually conducts (alone or with others) a clinical investigation Human subject - correct answer an individual who is or becomes a par...

-
Agritech 2 Certification Questions with 100% Correct Answers
- Exam (elaborations) • 10 pages • 2024
-
- $12.49
- + learn more
Agritech 2 Certification Questions with 100% Correct Answers Which event resulted in developing countries being able to produce higher-yielding, disease and insect-resistant varieties of small grain? - Correct Answer ️️ -Green Revolution Approximately how much of the food dollar goes to the producer? - Correct Answer ️️ - 16 cents What type of material is preferred for footwear for most workers because of its strength, durability, safety, and comfort? - Correct Answer ️️ -Lea...

-
ACRP-CP Certification Exam 2024/25 EDITION GUARANTEED GRADE A+
- Exam (elaborations) • 21 pages • 2024
-
- $10.99
- + learn more
ACRP-CP Certification Exam 2024/25 EDITION GUARANTEED GRADE A+ What would be the first priority for an investigator when a subject wishes to withdraw prematurely from the trial? Try to obtain the subject's reason for withdrawal. CRO recently switched from paper CRF to an EDC system. The EDC system must conform to the established requirements for Validation Accuracy Reliability Completeness Part of a sponsor's responsibility pertaining to electronic trial data handling is to maintain a...

-
ACRP CP FINAL EXAM LATEST 2023 REAL EXAM 150 QUESTIONS AND CORRECT ANSWERS|AGRADE
- Exam (elaborations) • 19 pages • 2024
-
- $13.49
- + learn more
What would be the first priority for an investigator when a subject wishes to withdraw prematurely from the trial? - ANSWER- Try to obtain the subject's reason for withdrawal. CRO recently switched from paper CRF to an EDC system. The EDC system must conform to the established requirements for: - ANSWER- Validation, accuracy, reliability, completeness Part of a sponsor's responsibility pertaining to electronic trial data handling is to - ANSWER- maintain an audit trail, data trail, and ...

-
CCRP Definitions with complete solutions | Latest 2023/2024
- Exam (elaborations) • 26 pages • 2023
- Available in package deal
-
- $11.99
- + learn more

That summary you just bought made someone very happy. Also get paid weekly? Sell your study resources on Stuvia! Discover all about earning on Stuvia


