Pennfoster College
Latest uploads at Pennfoster College. Looking for notes at Pennfoster College? We have lots of notes, study guides and study notes available for your school.
-
96
- 0
-
2
All courses for Pennfoster College
Latest notes & summaries Pennfoster College
Organic Chemistry ACS Final Exam Questions With Correct Verified Solutions A+ Graded Basicity rules - ANS -strong base: unstable/reactive (ED groups) -weak base: stable (EW groups/more EN/bigger) Chair conformation - ANS -more stable equatorial (slanted) -pointing up/pointing down=cis if both up -elimination: trans and diaxial Newman projection - ANS -gauche: substituent groups adjacent to each other -grab middle and turn to put in plane Enantiomers - ANS -not superimpos...
Organic Chemistry ACS Final Exam Questions And Answers All Verified By An Expert A+ Graded ketone aldehyde carboxylic acid ester
O-H and N-H IR shift - 3400 cm⁻¹ C-H SP³ IR shift - cm⁻¹ C-H SP₂ IR shift - cm⁻¹ C-H SP IR Shift - 3300 cm⁻¹ 3x bond C-C IR shift - 2100 cm⁻¹ C=O IR shift - 1700 cm⁻¹ C=C and C-C aromatic IR shift - 1600 cm⁻¹ Aromatic C-C IR shift - 1500 cm⁻¹ HNMR shift of methyl hydrogen - 1 ppm HNMR shift of hydrogen attached to SP2 carbon - 2 ppm HNMR shift of aromatic hydrogen - 7-8 ppm HNMR shift of hydrogen attached to SP3 carbon attached to a p...
C C A D B D B A C A B
What is the general formula for a carbohydrate? - Cx(H2O)y How do you determine the number of stereoisomers for a given carbohydrate? - 2^n where n= number of stereocenters Distinguish between the D and L designations for carbohydrates. - In the L form the hydroxy group is on the bottom left closest to the CH2OH group in the frame of a Fischer projection In the D form the OH is on the bottom right What are anomers? - Diastereomers formed by the cyclization of carbohydrates (*note they ...
Alcohol - Ketone - Amine - Ether - Amide - Ester - functional groups - Alkyl - An alkane missing a hydrogen Aromatic compound - Cyclic, planar, with every atom of the aromatic ring having a p orbital, while obeying hackles rule of 4n+2 pi electrons Vinyl - Hydrolysis Reaction - Carboxylic Acid - pKa ~ 5 Enantiomers - stereoisomers that are non super imposable mirror images Diastereomers - - Same molecular formula, same bond connections, NOT mirrors...
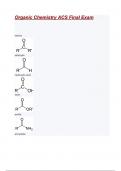
Functional group: alkane Functional group: alkene Functional group: alkyne Functional group: benzene ring (phenyl) Functional group: amine Functional group: alcohol Functional group: ether Functional group: alkyl halide Functional group: thiol Functional group: aldehyde Functional group: ketone Functional group: ester
-al -ol or hydroxy- -one -oic acid -oate -ether or alkoxy- Iodo- or Bromo- etc. -amide Amino- or -amine - Aldehyde Alcohol Ketone Carboxylic acid Ester Ether Alkyl halides Amide Amine Constitutional Isomer - A compound with the same atoms but different atoms bonded to each other Stereoisomers - Molecules with the same atoms differing only in 3D configuration Enantiomers - Stereoisomers with exactly opposite stereochemical designations at all stereogenic carbons Dia...
sp, linear - Which is the correct hybridization state and geometry for the carbon atom in HCN? sp, linear sp2, trigonal planar sp3, tetrahedral None of the above This structure has one positive charge and one negative charge. - The following structure has been drawn without formal charges. Which statement describes the missing formal charge(s)? This structure has one positive charge and one negative charge. This structure has one positive charge but no negative charges. This structure ...
Basicity rules - -strong base: unstable/reactive (ED groups) -weak base: stable (EW groups/more EN/bigger) Chair conformation - -more stable equitorial (slanted) -pointing up/pointing down=cis if both up -elimination: trans and diaxial Newman projection - -gauche: substituent groups adjacent to eachother -grab middle and turn to put in plane Enantiomers - -not superimposable mirror images -same besides stereochemistry Diastereomers - -mirror images but one stereocenter stays and...