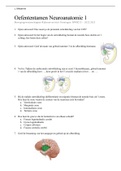
Exam (elaborations)
GCSE CHEMISTRY 8462/1H Paper 1 Higher Tier Mark scheme June 2020
- Module
- Chem
- Institution
- PEARSON (PEARSON)
GCSE CHEMISTRY 8462/1H Paper 1 Higher Tier Mark scheme June 2020 Version: 1.0 Final Mark Scheme *206G8462/1H/MS* Mark schemes are prepared by the Lead Assessment Writer and considered, together with the relevant questi...
[Show more]