Summary
Summary BTEC applied science/forensics unit 1 Chemistry: Percentage yield
- Institution
- PEARSON (PEARSON)
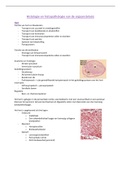
These are my notes on percentage yield and stoichiometry, complete with an example question and how to tackle it. This can be used for both BTEC applied science and forensics as the unit is the same, please leave a review! :)
[Show more]