Summary
Summary BTEC APPLIED SCIENCE UNIT 4 ASSIGNMENT B: (DISTINCTION)
- Institution
- PEARSON (PEARSON)
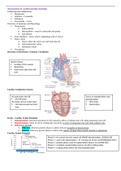
This coursework is for unit 4 , learning aim B and it was awarded distinction grade. This assignment can be bought as a guideline or as a skeleton to help you aim to get a distinction. Check out my store for other units and for unit bundle at discounts. Please contact me for any help.
[Show more]