Lecture notes
Carbohydrates, Lipids and risk factors
- Institution
- PEARSON (PEARSON)
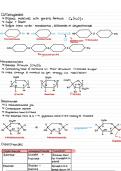
Describes the structure of carbohydrates and lips including the reactions and bonding types. Includes functions of certain disaccharides and polysaccharides. Descriptions of saturated and unsaturated lipids. All with useful diagrams and tables. All labelled n highlighted key terms .
[Show more]