Revision Material
Duration: 2nd – 8th March
Topic 3 Chemical Bonding
This topic describes the type, number and distribution of the fundamental particles which make up an atom and the impact of this on some atomic properties.
Learning outcomes
Candidates should be able to:
3.1 Ionic bonding (a) describe ionic bonding, using the examples of sodium chloride, magnesium oxide and
calcium fluoride, including the use of ‘dot-and- cross’ diagrams
3.2 covalent bonding and (a) describe, including the use of ‘dot-and-cross’ diagrams:
co-ordinate (dative 1. (i) covalent bonding, in molecules such as hydrogen, oxygen, chlorine, hydrogen
covalent) bonding chloride, carbon dioxide, methane, ethene
including shapes of 2. (ii) co-ordinate (dative covalent) bonding, such as in the formation of the ammonium ion
simple molecules and in the Al2Cl6 molecule
(b) describe covalent bonding in terms of orbital overlap, giving σ and π bonds, including the
concept of hybridisation to form sp, sp2 and sp3 orbitals (see also Section 14.3)
(c) explain the shapes of, and bond angles in, molecules by using the qualitative model of
electron-pair repulsion (including lone pairs), using as simple examples BF3 (trigonal planar),
CO2 (linear), CH4 (tetrahedral), NH3 (pyramidal), H2O (non-linear), SF6 (octahedral), PF5
(trigonal bipyramidal)
(d) predict the shapes of, and bond angles in, molecules and ions analogous to those specified in
3.2(c) (see also Section 14.3)
3.3 intermolecular forces, (a) describe hydrogen bonding, using ammonia and water as simple examples of molecules
electronegativity and containing N–H and O–H groups
bond properties (b) understand, in simple terms, the concept of electronegativity and apply it to explain the
properties of molecules such as bond polarity (see
also Section 3.3(c)), the dipole moments of molecules (3.3(d)) and the behaviour of oxides
with water (9.2(c))
(c) explain the terms bond energy, bond length and bond polarity and use them to compare the
reactivities of covalent bonds (see also Section 5.1(b)(ii))
(d) describe intermolecular forces (van der Waals’ forces), based on permanent and induced
dipoles, as in, for example, CHCl 3(l); Br2(l) and the liquid Group 18 elements
3.4 metallic bonding (a) describe metallic bonding in terms of a lattice of positive ions surrounded by delocalised
electrons
3.5 Bonding and physical (a) describe, interpret and predict the effect of different types of bonding (ionic bonding, covalent
properties bonding, hydrogen bonding, other intermolecular interactions, metallic bonding) on the
physical properties of substances
(b) deduce the type of bonding present from given information
(c) show understanding of chemical reactions in terms of energy transfers associated with the
breaking and making of chemical bonds
, TYPES OF BOND
Chemical Bonds (Strong bonds) ¨ ionic
¨ covalent
¨ detive covalent
¨ metallic
Physical bonds (weak bonds) ¨ induced dipole-dipole interactions (London Forces)
¨ permanent dipole-dipole interactions
(both the above are examples of Van der Waals’s Forces)
¨ hydrogen bonds
strong
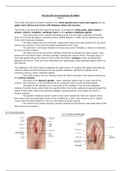
3.1 Ionic bonding and ionic lattice
describe ionic bonding, using the examples of sodium chloride, magnesium oxide and calcium fluoride, including the use of
‘dot-and- cross’ diagrams
ionic bond is the electrostatic attraction between oppositely charged ions.
Structure : giant ionic lattice, crystalline solids
Properties high melting and boiling points
(i) Common properties
melting point Very high A large amount of energy must be put in to overcome the strong electrostatic
attractions and separate the ions.
strength Very brittle Any dislocation leads to layers moving and similarly charged ions being next to
each other. The repulsion splits the crystal.
electrical • do not conduct electricity when solid - ions are held strongly in the lattice
• conduct electricity when molten or in aqueous solution - the ions become mobile and
conduction takes place.
solubility • insoluble in non-polar solvents
• soluble in water as it is a polar solvent and stabilises the separated ions
• energy is needed to overcome the electrostatic attraction and separate the ions
• stability is achieved by polar water molecules surrounding the ions
- Conducting electricity in molten state (aq) as it have free moveable ions
- High melting point / boiling point and hard
Cations and anions are held by strongly by ionic bond
Draw a ‘dot-and-cross’ diagram to show the arrangement of outer electrons present in a formula unit of Mg2Si. Assume
magnesium silicide is an ionic compound.
sodium chloride calcium fluoride
magnesium oxide