Samenvatting
Summary PSY1023 case 7 hunger and thirst
- Instelling
- Maastricht University (UM)
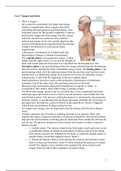
Case 7 from the course body and behaviour which is part of the bachelor psychology and advanced minor in psychology. I got a 9 for the exam myself.
[Meer zien]